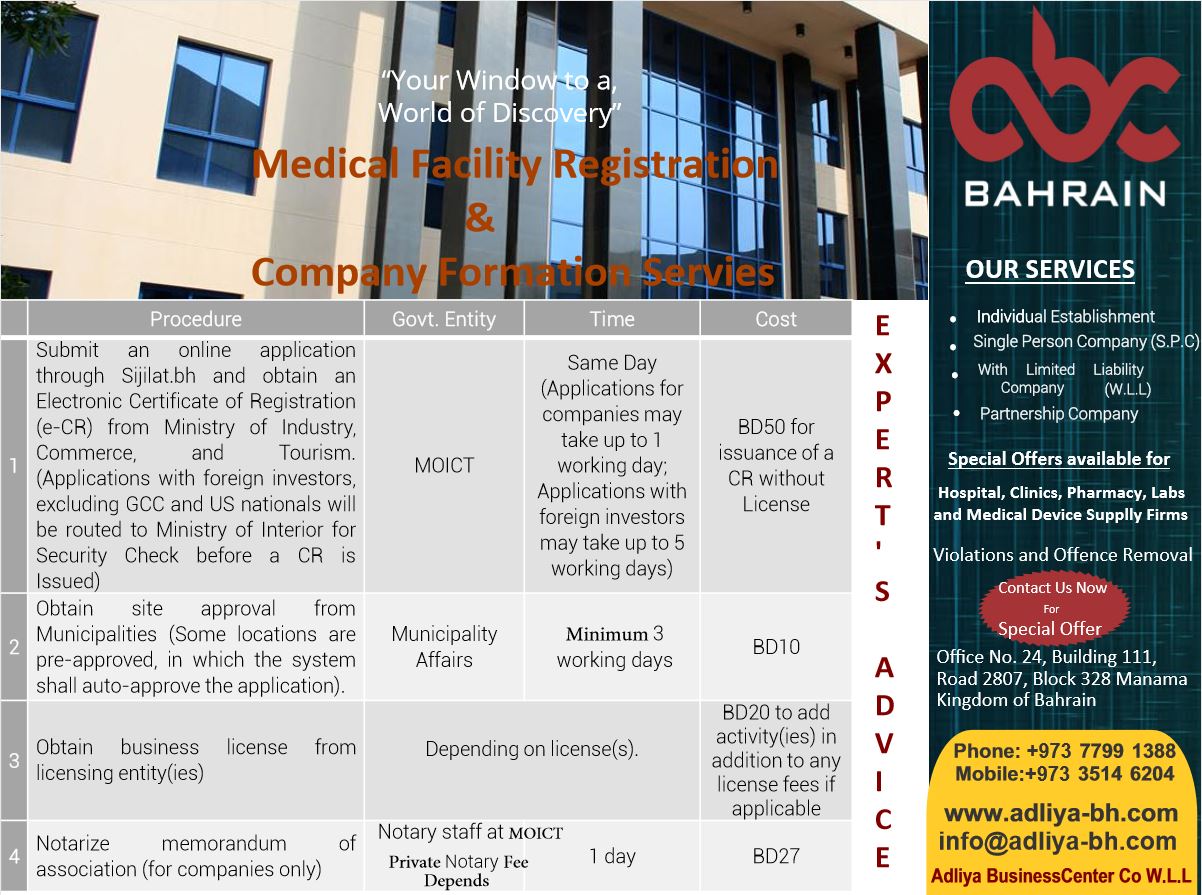
Company Formation
Company Formation Services for Medical Device Supply Companies in Bahrain, Oman, and Saudi Arabia
Adliya Business Center Co WLL (ABC) specializes in providing comprehensive company formation services tailored for medical device supply businesses in Bahrain, Oman, and Saudi Arabia. Our expertise in regulatory compliance and business setup ensures a smooth and efficient process for establishing your operations in these vital GCC markets.
In addition to medical device companies, we assist in the formation of various types of businesses, helping entrepreneurs navigate the complexities of local regulations. Whether you're looking to establish a limited liability company, a joint venture, or any other business structure, our team is dedicated to facilitating your success.
From selecting the appropriate legal structure to securing necessary licenses, we provide end-to-end solutions that position your business for growth in these dynamic regions. Partner with us to unlock your potential and confidently enter the GCC market.
- Business Registration and Licensing:
- Assistance with registering your company under the appropriate legal structure (e.g., LLC, WLL) in Bahrain, Oman, and Saudi Arabia.
- Guidance on obtaining necessary trade licenses specific to medical device supply.
- Regulatory Compliance:
- Support in meeting country-specific regulatory requirements, including approvals from:
- National Health Regulatory Authority (NHRA) in Bahrain.
- Ministry of Health (MOH) in Oman.
- Saudi Food and Drug Authority (SFDA) in Saudi Arabia.
- Ensuring compliance with importation, storage, and marketing standards.
- Support in meeting country-specific regulatory requirements, including approvals from:
Why Choose ABC?
- Expertise Across GCC Markets: With a deep understanding of the healthcare regulations in Bahrain, Oman, and Saudi Arabia, we streamline the setup process for medical device supply companies.
- Fast-Track Solutions: Benefit from our fast-track services for regulatory approvals and licensing to get your business operational quickly.
- Comprehensive Support: From registration to post-market surveillance compliance, we provide end-to-end support to ensure your success.
Bahrain:
- Assistance with NHRA licensing and compliance with new regulations mandating licenses for all medical devices marketed or imported into the country.
- Support for fast-track registration of devices already approved by reference countries like Saudi Arabia (SFDA), the US (FDA), or the EU.
Steps to Establish a Medical Device Supply Business:
- Business Registration: Register your company with Bahrain’s Ministry of Industry, Commerce, and Tourism (MOICT). Choose an appropriate business structure, such as a Limited Liability Company (LLC).
- NHRA Licensing: Obtain a facility license from NHRA, which is mandatory for importing and supplying medical devices.
- Medical Device Registration: Ensure all devices are registered with NHRA. This includes appointing an authorized representative and submitting technical documentation like quality assurance certificates, user manuals, and labeling in Arabic or English.
- Compliance with Import Regulations: Importation is a separate process requiring customs clearance and adherence to NHRA standards.
Key Advantages:
- Fast-track registration options through NHRA.
- Access to Bahrain’s growing healthcare infrastructure.
Oman:
Oman’s healthcare sector is rapidly expanding, with regulations managed by the Directorate General for Pharmaceutical Affairs and Drug Control under the Ministry of Health (MOH).
Steps to Establish a Medical Device Supply Business:
- Business Registration: Register your company with the Ministry of Commerce, Industry, and Investment Promotion (MOCIIP). Most investors opt for an LLC for flexibility in operations.
- Medical Equipment Supply License: Apply for a license from MOH by submitting essential documents like commercial registration certificates, product specifications, and warehouse approvals.
- Device Registration: Appoint an Omani authorized representative to handle regulatory compliance and submit technical documentation such as CE certificates, Free Sales Certificates, and Declaration of Conformity.
- Import Clearance: Obtain customs clearance for imported devices while ensuring compliance with health regulations.
Key Advantages:
- There is a growing demand for advanced medical devices due to modernization efforts.
- Opportunities in telemedicine and medical tourism sectors.
Saudi Arabia:
- Comprehensive support for SFDA Medical Device Marketing Authorization (MDMA).
- Assistance with appointing a Local Authorized Representative to manage regulatory obligations.
Saudi Arabia has one of the largest healthcare markets in the region, regulated by the Saudi Food and Drug Authority (SFDA).
Steps to Establish a Medical Device Supply Business:
- Facility License: Obtain a facility license from SFDA to legally import and market medical devices.
- Legal Representative Appointment: Appoint a local authorized representative or distributor responsible for regulatory compliance.
- Medical Device Marketing Authorization (MDMA): Register all medical devices with SFDA by submitting detailed technical files covering safety, efficacy, intended use, and risk classification.
- Post-Market Surveillance: Ensure ongoing compliance with SFDA standards through regular reporting and monitoring.
Key Advantages:
- Access to one of the largest healthcare markets in the Middle East.
- Alignment with international standards such as FDA and EU regulations.
Why Choose These Markets?
- Strong government investments in healthcare infrastructure across Bahrain, Oman, and Saudi Arabia.
- Increasing demand for specialized medical devices due to chronic diseases and advancements in technology.
- Opportunities for partnerships with hospitals, clinics, and government procurement programs.
Partner with ABC Today
Whether you’re an international manufacturer or a local entrepreneur looking to enter the medical device supply market in Bahrain, Oman, or Saudi Arabia, ABC is your trusted partner. Contact us today to simplify your company formation process and unlock opportunities in these thriving healthcare markets!
Have any Inquiry?
Contact Us

Company Address
Office: 24, Building: 111,
Road: 2807, Block: 328,
Area: Manama,
Kingdom Of Bahrain.